Multiple Choice
If the temperature of a gas triples,by what factor does the speed of a molecule with the average kinetic energy change?
A) It increases by a factor of 3.
B) It increases by a factor of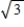 .
.
C) It increases by a factor of 3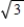 .
.
D) It increases by a factor of 1/3.
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q14: A helium-filled weather balloon has a 0.90-m
Q15: Suppose there are 2 moles of a
Q16: How many atoms are present in a
Q17: How many molecules are there in 14
Q18: A storeroom has a volume of 30
Q18: In the relationship ΔP ∝ ΔT ,what
Q21: If the average kinetic energy of the
Q22: A spherical air bubble originating from a
Q23: One way to heat a gas is
Q77: What is the mass of 2 moles