Multiple Choice
In an isothermal process where the pressure decreases from 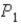
To 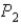
,does the internal energy of the system increase,decrease,or stay the same;and does the entropy of the system increase or decrease?
A) The internal energy decreases and the entropy increases.
B) The internal energy stays the same and the entropy increases.
C) The internal energy decreases and the entropy decreases.
D) The internal energy stays the same and the entropy decreases.
Correct Answer:

Verified
Correct Answer:
Verified
Q9: A heat engine operating between a pair
Q10: The surface of the Sun is at
Q11: A 2.0-mol ideal gas system is maintained
Q12: In an isobaric process 3.6 × 10<sup>4</sup>
Q16: A quantity of monatomic ideal gas expands
Q17: A gasoline engine with an efficiency of
Q18: A turbine takes in 1000 K steam
Q20: You have a PV diagram for a
Q28: The laws of thermodynamics can be related
Q59: Consider a Carnot cycle starting at the