Multiple Choice
Sample #1 is made from an isotope with decay constant 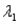
And sample #2 is made from an isotope with decay constant 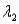
,where 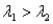
) Which of the following statements must be true?
A) The activity of sample #2 is greater than that of sample #1.
B) The activity of sample #1 is greater than that of sample #2.
C) The half-life exhibited for sample #1 is greater than that of sample #2.
D) The half-life exhibited for sample #2 is greater than that for sample #1
Correct Answer:

Verified
Correct Answer:
Verified
Q21: A pure sample of <sup>226</sup>Ra contains 3.0
Q22: An ancient building was known to have
Q23: What particle is emitted when <sup>236</sup>Pa decays
Q24: One of the nuclear reactions that occurs
Q25: If a fossil bone is found to
Q27: Tritium has a half-life of 12.3 years.How
Q28: Neglecting recoil of the gold nucleus,how much
Q29: Over the course of 6 hours,15% of
Q30: The energy released from a nuclear reaction
Q31: Tritium (<sup>3</sup>H)has a half-life of 12.3 years