Multiple Choice
A sample of pure carbon weighing 1.48 g was burned in an excess of air.The mass of carbon dioxide,the sole product,was 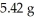
.In a second experiment,11.62 g of carbon dioxide was obtained.What mass of carbon was burned in the second experiment?
A) 42.6 g
B) 3.17 g
C) 3.54 g
D) 0.866 g
E) 11.6 g
Correct Answer:

Verified
Correct Answer:
Verified
Q47: Argon belongs to the _ group of
Q48: A cubic centimeter of lead weighs 11.35
Q49: Copper occurs in an isotopic mixture of
Q50: Robert Millikan determined the charge on an
Q51: How many atoms of sulfur are in
Q53: Choose the INCORRECT statement from those given
Q54: Atoms combine in small,whole-numbered ratios.
Q55: A 3.214 g sample of magnesium reacts
Q56: Dalton's atomic theory is based on several
Q57: Which statement below is true?<br>A)Metals gain electrons