Multiple Choice
With mass spectral data the ratio of the mass of 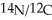
Was found to be 1.167.What is the mass of the 14N atom?
A) 14.017 u
B) 10.292 u
C) 14.004 u
D) 17.000 u
E) 14.007 u
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q103: How many moles of Fe is present
Q104: How many atoms of silicon are contained
Q105: The total numbers of neutrons,protons,and electrons in
Q106: A species that differs in charge from
Q107: 24.9 g of Mn is equivalent to
Q109: An isotope with mass number 81 has
Q110: Beta particles:<br>A)have a mass of 4 and
Q111: A new element is discovered.It has two
Q112: When decomposed chemically,73.0 grams of a sample
Q113: A 1.4 g sample of calcium is