Multiple Choice
A cation has 13 neutrons and 10 electrons.If it has a charge of +1,what is its correct symbol?
A) 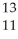
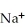
B) 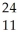
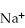
C) 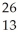
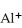
D) 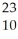
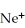
E) 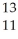
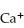
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q1: The atomic weight of chlorine is very
Q2: Which is the proper chemical symbol for
Q3: How many Cu atoms are present in
Q5: How many atoms of hydrogen are present
Q6: Different ratios of atoms produce different compounds.
Q7: Lead-204 has a relative abundance of 1.48%.What
Q8: What is the mass in grams of
Q9: An atom that has an atomic number
Q10: When a chemical reaction is carried out
Q11: Silver possesses two stable isotopes: <sup>107</sup>Ag (106.90