Multiple Choice
An anion has 45 neutrons and 36 electrons.If it has a -1 charge,what is its correct symbol?
A) 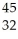
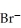
B) 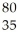
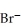
C) 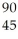
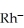
D) 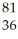
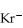
E) 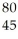
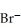
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q33: There are two stable isotopes of supposium
Q34: Write the appropriate symbol for the species
Q35: Atoms retain their identity during a chemical
Q36: A hypothetical element,E,has two stable isotopes.<br>E-38 =
Q37: Choose the INCORRECT statement.<br>A)The law of constant
Q39: What is the mass number of the
Q40: A cation has 28 neutrons and 21
Q41: A certain element contains eleven atoms of
Q42: An element has 5 stable isotopes.The mass
Q43: Iodine belongs to the _ group of