Multiple Choice
What is the atomic weight of an element if 4.00 grams of it contain 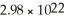
Atoms?
A) 20.2 u
B) 80.8 u
C) 19.7 u
D) 8.08 u
E) 2.02 u
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q65: How many protons (p)and neutrons (n)are in
Q66: Identify the chemical symbol of element Q
Q67: Write the symbol for the radioactive isotope
Q68: Cesium belongs to the _ group of
Q69: Choose the information a mass spectrometer is
Q71: An element has 5 stable isotopes.The mass
Q72: All atoms of a given element are
Q73: Lead has 4 stable isotopes with masses
Q74: What is the isotopic atomic mass of
Q75: J.J.Thomson suggested the "plum pudding" model of