Multiple Choice
What is the thickness in mm of the sheet 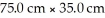
Formed by 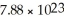
Atoms of lead? The density of lead is 11.35 g/cm3.
A) 9.10 mm
B) 0.0910 mm
C) 1.88 × 104 mm
D) 0.273 mm
E) 2.73 mm
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q90: Write the symbol for the most common
Q91: Rubidium possesses two stable forms and has
Q92: Which of the following is a correct
Q93: Choose the INCORRECT statement.<br>A)Gamma rays are bent
Q94: The total number of neutrons in an
Q96: Sodium is a member of the family
Q97: How many moles of lead are present
Q98: Magnesium has 3 stable isotopes with masses
Q99: All matter is composed of atoms.
Q100: The three naturally occurring isotopes of magnesium