Multiple Choice
To produce ferrocene one requires 2.33 g of C5H5 (65.0 g/mol) for every 1.00 g of Fe used (55.9 g/mol) .What is the value of x in the formula of ferrocene: 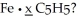
A) 1
B) 2
C) 3
D) 4
E) 5
Correct Answer:

Verified
Correct Answer:
Verified
Q73: What is the oxidation number of the
Q74: Which of the following is the correct
Q75: A compound has the following percentage composition,by
Q76: For an ionic compound,the term formula mass
Q77: A 10.0 g sample of bismuth tribromide,BiBr<sub>3</sub>,contains:<br>A)5.37
Q79: What mass of ammonia,N<sub> </sub>H<sub>3</sub>,contains the same
Q80: Which one of the following compounds contains
Q81: What is the mass percent of H<sub>2</sub>O
Q82: What is the formula of sodium hypochlorite?<br>A)NaCl<br>B)NaClO<br>C)NaClO<sub>2</sub><br>D)NaClO<sub>3</sub><br>E)NaClO<sub>4</sub>
Q83: A 200.0 g sample of pure MgCl<sub>2</sub>