Multiple Choice
What is the empirical formula of a compound that is 62.0% C,10.4% H,and 27.5% O by mass?
A) 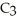
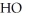
B) 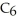
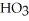
C) 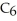
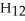
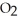
D) 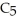
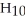
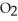
E) 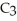
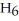
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q58: What is the oxidation number of the
Q59: What is the oxidation state of chlorine
Q60: What is the correct name for the
Q61: Combustion analysis of 1.200 g of an
Q62: A sample of pure lithium nitrate contains
Q64: The molecular formula for a sugar molecule
Q65: Choose the INCORRECT oxidation state.<br>A)Cl is +5
Q66: Choose the INCORRECT statement.<br>A)A structural formula shows
Q67: Which one of the following is NOT
Q68: From the following percent by mass compositions,determine