Multiple Choice
For the reaction 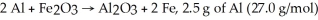
And 7.2 g of Fe2O3 (159.8 g/mol) produce 5.03 g of Fe (55.9 g/mol) .Calculate the extent of reaction ,x.
A) 0.362
B) 0.089
C) 0.045
D) 0.092
E) 0.405
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q37: How many millilitres of a 9.0 mol
Q38: Lithium and nitrogen react in a combination
Q39: What is the sum of the coefficients
Q40: How many grams of sulfuric acid,H<sub>2</sub>SO<sub>4</sub>,can be
Q41: Given the following reactions:<br>Fe + Br<sub>2</sub> →
Q43: How many moles of H<sub>3</sub>PO<sub>4</sub> are produced
Q44: What is the concentration (M)of sodium ions
Q45: What is the molarity of 10.9 g
Q46: Which of the following represents a 1.00
Q47: For the reaction <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB5343/.jpg" alt="For the