Multiple Choice
The chemical reaction occurring during the discharge of a lead storage battery can be represented by the equation: 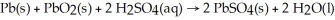
What mass of lead sulfate would result from the complete reaction of 41.4 g of lead?
A) 57.6 g
B) 60.5 g
C) 105 g
D) 115 g
E) 121 g
Correct Answer:

Verified
Correct Answer:
Verified
Q160: What is the stoichiometric coefficient for oxygen
Q161: Iron metal reacts with chlorine gas as
Q162: When silver nitrate reacts with barium chloride,silver
Q163: One source of iodine is sodium iodate.Iodine
Q164: If the percent yield for the following
Q165: What is the sum of the coefficients
Q166: For the reaction <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB5343/.jpg" alt="For the
Q168: If the reaction of phosphate ion with
Q169: What thickness (in cm)is the silicon block
Q170: 52.5 mL of a solution were diluted