Multiple Choice
What mass of oxygen gas would be consumed by the complete combustion of 7.5 g of a mixture of propane (C3H8) and butane (C4H10) in the mole ratio of 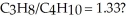
A) 3.9 g
B) 11 g
C) 14 g
D) 21 g
E) 27 g
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q154: "Washing soda" (sodium carbonate)may be used to
Q155: How many millilitres of a stock solution
Q156: Gases emitted during volcanic activity often contain
Q157: You have 10.00 L of a 0.350
Q158: How many grams of CrSO<sub>4</sub> will be
Q160: What is the stoichiometric coefficient for oxygen
Q161: Iron metal reacts with chlorine gas as
Q162: When silver nitrate reacts with barium chloride,silver
Q163: One source of iodine is sodium iodate.Iodine
Q164: If the percent yield for the following