Multiple Choice
Lead ions can be precipitated from aqueous solutions by the addition of aqueous iodide: 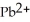
(aq) + 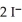
(aq) → 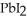
(s)
Lead iodide is virtually insoluble in water so that the reaction appears to go to completion.How many millilitres of 3.550 mol L-1 HI(aq) must be added to a solution containing 0.700 mol of 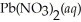
To completely precipitate the lead?
A) 2.54 × 10-3 mL
B) 394 mL
C) 197 mL
D) 0.197 mL
E) 0.394 mL
Correct Answer:

Verified
Correct Answer:
Verified
Q89: In which of the following pairs is
Q90: The neutralization of 25.0 mL of 0.24
Q91: What concentration of Fe<sub>2</sub>(SO<sub>4</sub>)<sub>3</sub>(aq)is required for the
Q92: Which of the following compounds is quite
Q93: An insoluble compound will dissolve to an
Q95: How many electrons are transferred when the
Q96: The substance BaCl<sub>2</sub> is a:<br>A)salt<br>B)weak acid<br>C)strong base<br>D)weak
Q97: Balance the following oxidation-reduction reaction in acid
Q98: The substance NH<sub>3</sub> is a:<br>A)weak acid<br>B)strong acid<br>C)salt<br>D)weak
Q99: Balance the following equation in basic solution:<br>Br<sub>2</sub>