Multiple Choice
Silver ions can be precipitated from aqueous solutions by the addition of aqueous chloride: 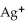 (aq) +
(aq) + 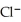 (aq) → AgCl(s)
(aq) → AgCl(s)
Silver chloride is virtually insoluble in water so that the reaction appears to go to completion.How many grams of solid NaCl must be added to 25.0 mL of 0.366 mol L-1 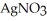 (aq) solution to completely precipitate the silver?
(aq) solution to completely precipitate the silver?
A) 9.15 × 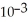 g
g
B) 1.57 × 10-4 g
C) 0.535 g
D) 0.157 g
E) 6.39 × 103 g
Correct Answer:

Verified
Correct Answer:
Verified
Q1: Which of the following represents a disproportionation
Q2: In which of the following pairs is
Q3: Which of the following represents a neutralization
Q5: Gold does not react with either nitric
Q6: Which of the compounds H<sub>2</sub>C<sub>2</sub>O<sub>4</sub>,Ca(OH)<sub>2</sub>,KOH,and HI behave
Q7: Identify the reducing agent in the following
Q8: Which one of the following compounds is
Q9: When 280 mL of 1.50 × 10<sup>-4
Q10: The mixing of which pair of reactants
Q11: Choose the INCORRECT statement.<br>A)Most molecular compounds are