Multiple Choice
In the reaction 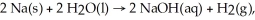
How many liters of hydrogen at STP are produced from 50.0 grams of sodium 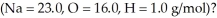
A) (55.0/18.0) (22.4) L
B) (50.0/23.0) (22.4/2) L
C) (50.0/23.0) (2) (22.4) L
D) (50.0/23.0) (22.4) L
E) (55.0/23.0) (22.4) L
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q92: In the reaction:<br>2 Al(s)+ 6 HCl(aq)→ 2
Q93: A sample of gas weighs 0.250 g
Q94: A 31.4 mL sample of nitrogen gas
Q95: Gases tend to behave ideally at:<br>A)low temperature
Q96: 53.5 g of an ideal gas of
Q98: Assuming ideal gas behavior,which of the following
Q99: A 4.0 L sample of N<sub>2</sub>(g)at 760
Q100: Which of the following gases has the
Q101: What is the average speed (actually the
Q102: The mole fraction of nitrous oxide in