Multiple Choice
The complete combustion of octane,a component of gasoline,is represented by the equation:
2 C8H18(l) + 25 O2(g) → 16 CO2(g) + 18 H2O(l)
How many liters of CO2(g) ,measured at 63.1 °C and 688 mmHg,are produced for every gallon of octane burned? 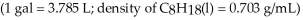
A) 7.48 L
B) 7.11 × 102 L
C) 5.68 × 103 L
D) 5.68 L
E) 1.07 × 103 L
Correct Answer:

Verified
Correct Answer:
Verified
Q50: The volume correction term in the van
Q51: Of the following gases,the one with the
Q52: The mutual attraction of gas molecules is
Q53: Pressure is a force per unit area
Q54: When one volume of CO reacts with
Q56: According to the kinetic-molecular theory of gases:<br>A)gaseous
Q57: Gas densities depend strongly on gas temperature
Q58: If CO<sub>2</sub> and NH<sub>3</sub> are allowed to
Q59: The statement,"For a fixed mass of gas
Q60: A 34.8 mL sample of an unknown,water-insoluble