Multiple Choice
Calculate the enthalpy change for the following reaction at 25 °C.The value of ΔfH° in kJ/mol is given below each species: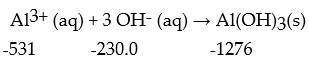
A) -515 kJ/mol
B) -55 kJ/mol
C) -975 kJ/mol
D) -1120 kJ/mol
E) -2040 kJ/mol
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q1: Combine the reactions:<br>P<sub>4</sub>(s)+ 6 Cl<sub>2</sub>(g)→ 4 PCl<sub>3</sub>(g)Δ<sub>r</sub>H°<sub>298</sub>
Q2: A 12-inch diameter ball of pure cobalt
Q4: The standard heat of formation of solid
Q5: A 50.0 g sample of liquid water
Q6: Enthalpy is defined as:<br>A)the heat of combustion<br>B)the
Q7: An expansion of gas by a system
Q8: The complete combustion of 1 mole of
Q9: The final temperature when 150 mL of
Q10: Lead,water,sulfur and arsenic have specific heats of
Q11: Calculate the standard enthalpy of formation of