Multiple Choice
The complete electron configuration of phosphorous,element 15,is:
A) 1s22s22p63s23p3
B) 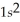
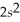 2p103s1
2p103s1
C) 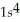
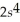 2p63s1
2p63s1
D) 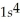 2s42p7
2s42p7
E) 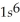 2s62p23s1
2s62p23s1
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q82: What is the general valence-electron ground-state electron
Q83: To what uncertainty,in pm,can the position of
Q84: Calculate the energy in kJ/mol of light
Q85: The Bohr theory explains that an emission
Q86: [Kr]5s<sup>2</sup>4d<sup>10</sup>5p<sup>5</sup> is the ground state electronic configuration
Q88: The Bohr theory explains that an emission
Q89: A radiation detector exposed to sunlight records
Q90: The possible values of the magnetic quantum
Q91: What is the total number of orbitals
Q92: A radiation detector exposed to sunlight records