Multiple Choice
The ion represented as 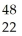 Ti4+ has:
Ti4+ has:
A) 22 electrons
B) 48 neutrons
C) 26 protons
D) 4 3d electrons
E) 0 4s electrons
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q48: Which of the following species will have
Q49: Which of the following reactions gives a
Q50: Which of the following pairs of elements
Q51: Can alkali metal atoms form negative ions
Q52: Which of the following could never be
Q54: Which of the following has the smallest
Q55: The energy difference between atomic cesium and
Q56: What is the general valence electronic configuration
Q57: List in order of increasing number of
Q58: The transition elements refer to what group