Multiple Choice
Write a Lewis structure for sodium iodide.
A) 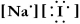
B) 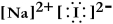
C) 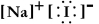
D) Na-I
E) 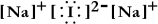
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q85: What is the molecular shape of BrF<sub>5</sub>?<br>A)linear<br>B)square
Q86: After drawing the Lewis dot structure for
Q87: The core electrons are called valence electrons.
Q88: How many "dots" should be shown for
Q89: An expanded octet may occur:<br>A)in the 1st
Q91: After drawing the Lewis dot structure for
Q92: The compound ClF<sub> </sub> contains:<br>A)ionic bonds<br>B)nonpolar covalent
Q93: How many lone pairs of electrons are
Q94: Given the bond enthalpies I-Cl (209),H-H (435),H-I
Q95: After drawing the Lewis dot structure of