Multiple Choice
For the molecule 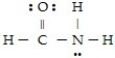
A) the hybridization for C is sp3
B) the hybridization for N is sp2
C) the hybridization for O is sp3
D) N is not hybridized
E) the hybridization for C is sp2
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q17: What are the F-Po-F bond angles in
Q18: Choose the INCORRECT statement about AsF<sub>6</sub><sup>-</sup>.<br>A)The hybridization
Q19: According to the principles of VSEPR applied
Q20: Using the VSEPR model,the electron-group geometry of
Q21: How many σ- and π-bonds,respectively,are there in
Q23: According to MO theory,all of the following
Q24: How many σ bonds and how many
Q25: What would be the hybridization of the
Q26: According to VB theory,sigma bonds are:<br>A)always present
Q27: According to the valence bond theory,in the