Multiple Choice
Which statement is INCORRECT about molecular orbital theory?
A) The number of molecular orbitals produced is equal to the number of atomic orbitals combined.
B) Each pair of sigma molecular orbitals is a bonding orbital and an antibonding orbital.
C) The antibonding orbital is at a lower energy than the bonding orbital.
D) The bond order (BO) is 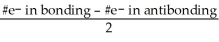 .
.
E) Hund's rule says that each orbital of identical energy has one electron before pairs are formed.
Correct Answer:

Verified
Correct Answer:
Verified
Q71: The hybrid orbital set used by the
Q72: For XeF<sub>4</sub>,what are the dipole moment orientation,hybridization
Q73: Unhybridized p orbitals must be present for
Q74: Using the VSEPR model,the molecular geometry of
Q75: Which of the pairs of molecules below
Q77: Using the VSEPR model,the molecular geometry of
Q78: What is the hybridization of the S
Q79: For BeCl<sub>2</sub>,the dipole moment of the molecule,hybridization
Q80: Choose the INCORRECT statement about H<sub>3</sub>O<sup>+</sup>.<br>A)There is
Q81: If the wave functions describing the 2s