Multiple Choice
According to MO theory,which is the INCORRECT statement for 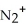 ?
?
A) The BO is 2.5.
B) There is one unpaired electron.
C) The σ2p orbital has one electron in it.
D) The molecule is diamagnetic.
E) There are 9 electrons in the molecular orbitals.
Correct Answer:

Verified
Correct Answer:
Verified
Q4: Using the VSEPR model, the molecular geometry
Q61: Using the VSEPR model,the electron-group geometry of
Q62: What geometric arrangement of charge clouds is
Q63: What is the molecular geometry of NCl<sub>3</sub>?<br>A)T-shaped<br>B)tetrahedral<br>C)trigonal
Q64: Which hybrid orbitals are impossible in electrically
Q65: Which of the following best describes ClF<sub>2</sub><sup>-</sup>?
Q67: The extra stability that a molecule gains
Q69: Which of the following statements is INCORRECT?<br>A)A
Q70: Choose the INCORRECT statement about PCl<sub>5</sub>.<br>A)There are
Q71: The hybrid orbital set used by the