Multiple Choice
One resonance structure of N2O is 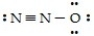
The hybridized atomic orbitals of the central nitrogen atom which are consistent with this structure are ________.
A) four sp3 orbitals
B) three sp2 orbitals and a p orbital
C) two sp2 orbitals and two sp orbitals
D) two sp orbitals and two p orbitals
E) one sp orbital and two p orbitals
Correct Answer:

Verified
Correct Answer:
Verified
Q6: Choose the INCORRECT statement about CO<sub>2</sub>.<br>A)There are
Q7: The valence-bond method describes covalent bonding as
Q8: Which of the following species is paramagnetic?<br>A)B<sub>2</sub><br>B)C<sub>2</sub><br>C)N<sub>2</sub><br>D)CO
Q9: Choose the INCORRECT statement about NO<sub>2</sub><sup>+</sup>.<br>A)There is
Q10: Which of the following carbon molecules has
Q13: The valence-bond method provides energy information about
Q14: Which statement regarding VB theory is INCORRECT?<br>A)A
Q15: The structure of aspirin is given below.(Note
Q16: For the molecule <br><img src="https://d2lvgg3v3hfg70.cloudfront.net/TB5343/.jpg" alt="For the
Q89: Using the VSEPR model, the molecular geometry