Multiple Choice
According to the phase diagram given,which of the following statements is INCORRECT? 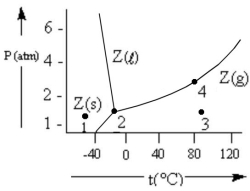
A) At the temperature and pressure of point 2,substance Z exists as a three-phase equilibrium system.
B) At the temperature and pressure of point 3,substance Z exists as a one-phase gaseous system.
C) If the Z(s) = Z(l) = Z(g) system is maintained at the temperature of point 2 while pressure is decreased,more Z will vaporize.
D) If liquid Z is maintained at the pressure of point 4 while the temperature is decreased to 30 °C,the liquid will vaporize.
E) The existence of liquid Z at -50 °C and 2 atm represents the metastable condition of "supercooling."
Correct Answer:

Verified
Correct Answer:
Verified
Q40: Which has the smallest dipole-dipole forces?<br>A)CH<sub>3</sub>Cl<sub> </sub><br>B)HBr<sub>
Q41: Consider a parallelepiped with all edges being
Q42: Which of the following compounds exhibits only
Q43: The heat of vaporization of water at
Q44: A crystal does not conduct electricity,yet its
Q46: Liquid and vapor phases of a substance
Q47: Surface tension is thought to be due
Q48: Given the data below,determine the molar enthalpy
Q49: Below are given the Lewis structures of
Q50: The enthalpy of condensation is equal to,but