Multiple Choice
According to the phase diagram given,which of the following is INCORRECT? 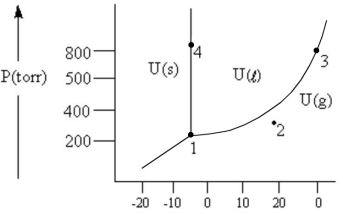
A) At the temperature and pressure of point 1,substance U exists as a three-phase equilibrium system.
B) At the temperature and pressure of point 2,substance U exists as a one-phase gaseous system.
C) At the temperature and pressure of point 3,substance U exists as a two-phase system.
D) If the U(s) ⇔ U(l) system is maintained at the temperature of point 4 while pressure is decreased steadily to about 300 Torr,more U will freeze.
E) There are no conditions of temperature and pressure under which solid U will vaporize without melting first.
Correct Answer:

Verified
Correct Answer:
Verified
Q60: Which of the following ionic compounds should
Q61: Which type of bonding does Sr form
Q62: Which combination of "type of solid" and
Q63: The process in which a gas is
Q64: How many H<sup>-</sup> ions are around each
Q66: Given the data below,determine the normal boiling
Q67: Which of the following forms an ionic
Q68: List the following ionic compounds in order
Q69: Gold crystallizes in a face-centred cubic structure.What
Q70: Which of the following statements concerning molecules