Multiple Choice
According to the phase diagram given,which of the following statements is INCORRECT? 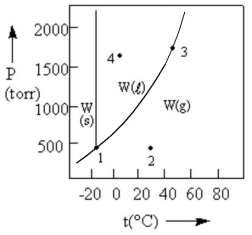
A) At the temperature and pressure of point 1,W exists as a three-phase equilibrium system.
B) At the temperature of point 2,a pressure of 500 Torr will cause W to liquefy.
C) If the system is maintained at the temperature of point 3 while pressure is decreased,more W will vaporize.
D) If W is maintained at the pressure of point 4 while the temperature is increased to 80 °C,the liquid will vaporize.
E) The existence of liquid W at -40 °C and 500 Torr represents the metastable condition of "supercooling."
Correct Answer:

Verified
Correct Answer:
Verified
Q28: A 0.90 g sample of liquid water
Q29: The phenomenon in which a steel needle
Q30: CH<sub>4 </sub>probably has a lower boiling point
Q31: Under which of the following conditions will
Q32: The heat of fusion for napthalene (C<sub>10</sub>H<sub>8</sub>)is
Q34: The property that causes water to have
Q35: Which of the substances below would produce
Q36: Given the following information,calculate ΔH° (in kcal
Q37: A liquid has a molar heat of
Q38: Vaporization occurs more readily with:<br>A)increased temperature,increased surface