Multiple Choice
For the reaction,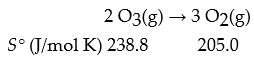
What is ΔrS°?
A) 137.4 J/mol ∙ K
B) -137.4 J/mol ∙ K
C) 33.8 J/mol ∙ K
D) 171.2 J/mol ∙ K
E) -33.8 J/mol ∙ K
Correct Answer:

Verified
Correct Answer:
Verified
Q34: An increase in entropy is observed when
Q35: If ΔG < 0 for a reaction,then
Q36: A spontaneous process:<br>A)will happen quickly<br>B)releases large amounts
Q37: The chemical potential of a substance,m,represents the
Q38: The following reaction is endothermic.<br>2 NH<sub>3</sub>(g)→ N<sub>2</sub>(g)+
Q40: For the reaction PCl<sub>5</sub> (g)⇌ PCl<sub>3</sub> (g)+
Q41: Consider the reaction:<br><img src="https://d2lvgg3v3hfg70.cloudfront.net/TB5343/.jpg" alt="Consider the reaction:
Q42: A microstate is a specific microscopic configuration
Q43: Consider the following reaction:<br><img src="https://d2lvgg3v3hfg70.cloudfront.net/TB5343/.jpg" alt="Consider the
Q44: Under which of the following conditions would