Multiple Choice
The enthalpy change for converting 1.00 mol of ice at -50.0 °C to water at 70.0 °C is  The specific heats of ice,water,and steam are
The specific heats of ice,water,and steam are 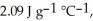
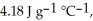 And
And 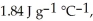 Respectively.For
Respectively.For  O,
O, 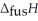 = 6.01 kJ mol-1,and
= 6.01 kJ mol-1,and 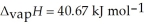 .
.
A) 12.28
B) 6.41
C) 13.16
D) 7154
E) 9.40
Correct Answer:

Verified
Correct Answer:
Verified
Q89: What is Δ<sub>r</sub>G° at 25 °C?<br>CO(g)+ 2
Q90: What is Δ<sub>r</sub>G°?<br><img src="https://d2lvgg3v3hfg70.cloudfront.net/TB5343/.jpg" alt="What is Δ<sub>r</sub>G°?
Q91: ?<sub>r</sub>G ? is independent of temperature.
Q92: Calculate Δ<sub>r</sub>G° for the reaction Cu(s)+ H<sub>2</sub>O(g)→
Q93: What is Δ<sub>r</sub>G° at 25 °C?<br>2O<sub>3</sub>(g)→ 3
Q95: For the reaction I<sub>2</sub>(s)+ Cl<sub>2</sub>(g)→ 2 ICl(g),Δ<sub>r</sub>H
Q96: Predict whether ΔS is positive or negative
Q97: Which of the following best expresses the
Q98: The entropy of a system always increases
Q99: A spontaneous process will occur only if