Multiple Choice
The fluorocarbon 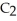
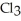
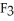 Has a normal boiling point of 47.6 °C.The specific heats of
Has a normal boiling point of 47.6 °C.The specific heats of 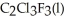 And
And 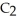
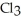
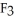 (g) are 0.91 J g-1 °C-1 and 0.67 J g-1 °C-1,respectively.The heat of vaporization of the compound is 27.49 kJ mol-1.The heat required to convert 50.0 g of the compound from the liquid at
(g) are 0.91 J g-1 °C-1 and 0.67 J g-1 °C-1,respectively.The heat of vaporization of the compound is 27.49 kJ mol-1.The heat required to convert 50.0 g of the compound from the liquid at 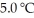 To the gas at 80.0 °C is ________ kJ.
To the gas at 80.0 °C is ________ kJ.
A) 8.19
B) 1454
C) 30.51
D) 3031
E) 10.36
Correct Answer:

Verified
Correct Answer:
Verified
Q72: If the vapor pressure of water in
Q73: Ethyl chloride,C<sub>2</sub>H<sub>5</sub>Cl,is used as a local anesthetic.It
Q74: The value of Δ<sub>f</sub>G at 100.0 °C
Q75: In a reversible reaction,a forward spontaneous reaction
Q76: Which one of the following would be
Q78: Which of the following relations is true
Q79: An increase or a decrease in the
Q80: Predict whether ΔS is positive or negative
Q81: For the reaction PCl<sub>5</sub>(g)→ PCl<sub>3</sub>(g)+ Cl<sub>2</sub>(g)at 298
Q82: The maximum quantity of energy available for