Multiple Choice
A solution is prepared by dissolving 16.2 g of benzene ( C6H6) in 282 g of carbon tetrachloride (CCl4) The concentration of benzene in this solution is ________ molal.The molar masses of C6H6 And CCl4 Are 78.1 g mol -1 And 154 g mol-1 Respectively.
A) 7.36 × 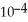
B) 0.736
C) 0.102
D) 0.0543
E) 5.43
Correct Answer:

Verified
Correct Answer:
Verified
Q109: An aqueous solution containing 12.0% MgCl<sub>2</sub> by
Q110: Which of the following is a correct
Q111: Given that the vapor pressure of pure
Q112: The freezing point of pure benzene was
Q113: The concentration unit used in determining freezing
Q115: Exactly 375.0 mg of an unknown protein
Q116: Which compound is likely to be the
Q117: Solutions are made that contain 0.10 moles
Q118: 100 mL of LiNO<sub>3</sub>(aq),0.241 M is mixed
Q119: Which of the following organic substances is