Multiple Choice
At 20 °C,a 0.376 mol L-1 aqueous solution of ammonium chloride has a density of 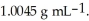 What is the mass % of ammonium chloride in the solution?
What is the mass % of ammonium chloride in the solution?
The formula weight of 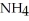 Cl is
Cl is 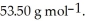
A) 0.381
B) 0.705
C) 0.374
D) 2.68
E) 2.00
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q48: If a solution containing 4 mols of
Q49: Which of the following statements about ideal
Q50: A sweetened cup of coffee is an
Q51: For the formation of an ideal solution
Q52: The concentration unit used in Raoult's Law
Q54: Which of the following is a true
Q55: What volume of an aqueous solution of
Q56: The solubility of CO in water at
Q57: In the solution,a solute is present in
Q58: Which compound is likely to be the