Multiple Choice
What is the freezing point of a solution of 7.15 g MgCl2 in 100 g of water?
Kf for water is 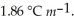
A) -0.140 °C
B) -1.40 °C
C) -2.80 °C
D) -4.18 °C
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q77: An aqueous solution contains 64.0 g of
Q78: What molality of pentane is obtained by
Q79: A mixture of benzene and toluene has
Q80: Solutions are made that contain 0.1 moles
Q81: A solution component present in lesser quantity
Q83: A magnesium sulfate heptahydrate solution,which is 18.00%
Q84: The solubility of CO in water at
Q85: A KCl solution is prepared by dissolving
Q86: What mass in grams of a molecular
Q87: What is the mole fraction of I<sub>2</sub>