Multiple Choice
An aqueous solution has a normal boiling point of 103.0 °C.What is the freezing point of this solution?
For water 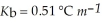 And
And 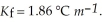
A) -0.82 °C
B) -3.0 °C
C) -3.6 °C
D) -11 °C
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q58: Which compound is likely to be the
Q59: An azeotropic mixture is a:<br>A)mixture of two
Q60: A solution of LiCl in water is
Q61: What is the molarity of a saturated
Q62: How many moles of ethylene glycol must
Q64: A solution prepared by dissolving 4.00 g
Q65: A solution that can dissolve no more
Q66: An isotonic solution will produce an osmotic
Q67: The freezing point of pure benzene was
Q68: We wish to lower the freezing point