Multiple Choice
A solution is prepared by dissolving 7.00 g of glycerin ( C3H8O3) in 201 g of ethanol 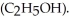 The freezing point of the solution is ________ °C.The freezing point of pure ethanol is
The freezing point of the solution is ________ °C.The freezing point of pure ethanol is 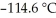 .The molal freezing point depression constant (
.The molal freezing point depression constant ( 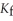 ) for ethanol is
) for ethanol is 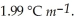 The molar masses of glycerin and of ethanol are 92.1 g mol-1 and 46.1 g mol-1,respectively.
The molar masses of glycerin and of ethanol are 92.1 g mol-1 and 46.1 g mol-1,respectively.
A) -121.3
B) 0.752
C) -107.9
D) -113.8
E) -115.4
Correct Answer:

Verified
Correct Answer:
Verified
Q1: At 20 °C,a 2.32 mol L<sup>-1</sup> aqueous
Q2: At a given temperature the vapour pressures
Q3: Nitrogen gas has a Henry's law constant
Q5: Moles of solute per mole of solution
Q6: Determine the osmotic pressure at 25 °C
Q7: What is the mole fraction of ethanol
Q8: What is the osmotic pressure in mmHg
Q9: 138.0 grams of ethanol (46.0 g/mol),99.0 grams
Q10: Which of the following compounds has the
Q11: A 1.38 M solution of nitric acid