Multiple Choice
Consider the following equilibrium reaction: PCl3(g) + Cl2(g) ⇌ PCl5(g) Kc = 96.2 The equilibrium constant for the decomposition of PCl5(g) to PCl3(g) and Cl2(g) is ________.
A) -Kc
B) 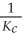
C) 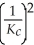
D) 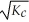
E) 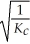
Correct Answer:

Verified
Correct Answer:
Verified
Q64: A mixture containing 0.392 M A(g)and 0.452
Q65: Consider the following chemical reaction at equilibrium:<br>2
Q66: The decomposition of ammonia is 2 NH<sub>3</sub>(g)→
Q67: 2.5 moles H<sub>2</sub>O and 100 g of
Q68: Given the following:<br>I.N<sub>2</sub>O(g)+ 1/2 O<sub>2</sub>(g)⇌ 2 NO(g)K<sub>c</sub>
Q70: At a certain temperature,nitogen and hydrogen react
Q71: Given that the equilibrium concentrations of [N<sub>2</sub>]
Q72: For a solute (X)aq,in an ideal aqueous
Q73: The equilibrium constant,K<sub>p</sub>,equals 3.40 at 25 °C
Q74: For the reaction: 2 SO<sub>2</sub>(g)+ O<sub>2</sub>(g)⇌ 2