Multiple Choice
Write the equilibrium constant expression for the following reaction:
6 CO2(g) + 6 H2O(l) ⇌ C6H12O6(s) + 6 O2(g)
A) Kc = 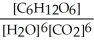
B) Kc = 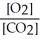
C) Kc = 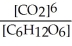
D) Kc = 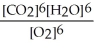
E) Kc = 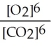
Correct Answer:

Verified
Correct Answer:
Verified
Q37: Which factor influences the value of the
Q38: In an equilibrium process,the concentrations of products
Q39: For the reaction: 3 Fe(s)+ 4 H<sub>2</sub>O(g)⇌
Q40: For the production of NO<sub>2</sub>,K<sub>c</sub> = [NO<sub>2</sub>]<sup>2</sup>/[NO]<sup>2</sup>[O<sub>2</sub>].At
Q41: What is the relationship between K<sub>p</sub> and
Q43: Consider the equilibrium system: N<sub>2</sub>O<sub>4</sub>(g)⇌ 2 NO<sub>2</sub>(g)for
Q44: In a reaction at equilibrium involving only
Q45: What is the value for K<sub>c</sub> for
Q46: For the decomposition of ammonium carbamate<br>NH<sub>4</sub>(NH<sub>2</sub>CO<sub>2</sub>)(s)⇌ 2
Q47: For the reaction:<br>PCl<sub>3</sub>(g)+ Cl<sub>2</sub>(g)⇌ PCl<sub>5</sub>(g)at 70.5 °C,K<sub>p</sub>