Multiple Choice
At 900.0 K,the equilibrium constant (Kp) for the following reaction is 0.345: 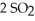 +
+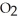 (g) →
(g) → 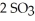 (g)
(g)
At equilibrium,the partial pressure of 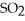 Is 35.0 bar and that of
Is 35.0 bar and that of 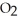 Is 15.9 bar.The partial pressure of
Is 15.9 bar.The partial pressure of 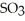 Is ________ bar.
Is ________ bar.
A) 82.0
B) 4.21 × 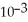
C) 192
D) 6.20 × 10-4
E) 40.2
Correct Answer:

Verified
Correct Answer:
Verified
Q55: Cyclohexane,C<sub>6</sub>H<sub>12</sub>,undergoes a molecular rearrangement in the presence
Q56: Consider the following reversible reaction:<br>POCl<sub>3</sub>(g)⇌ POCl(g)+ Cl<sub>2</sub>(g)K<sub>c</sub>
Q57: At a certain temperature,K<sub>c</sub> = 0.0500 and
Q58: For the reaction: 3 Fe(s)+ 4 H<sub>2</sub>O(g)⇌
Q59: One method to aid in working out
Q61: At 35 °C,K<sub>p</sub> = 0.315 for the
Q62: Choose the correct statement about a container
Q63: For the reaction: CH<sub>4</sub>(g)+ 2 H<sub>2</sub>O(g)⇌ CO<sub>2</sub>(g)+
Q64: A mixture containing 0.392 M A(g)and 0.452
Q65: Consider the following chemical reaction at equilibrium:<br>2