Multiple Choice
The pH of an aqueous solution at 25.0 °C is 10.55.What is the molarity of 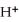
In this solution?
A) 2.8 × 10-11
B) 3.5 × 10-4
C) 3.3
D) 1.1 × 10-13
E) 3.5 × 1010
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q77: Which Bronsted-Lowry acid is not considered to
Q78: The base-dissociation constant of ethylamine ( <img
Q79: What is the pH of an aqueous
Q80: Choose the Br∅nsted-Lowry acids and bases in
Q81: What is the hydronium ion concentration of
Q83: Choose the INCORRECT statement.The term pH:<br>A)refers to
Q84: Calculate the hydronium ion concentration in an
Q85: An aqueous solution at 25.0 °C contains
Q86: A 250.0 ml sample of gaseous hydrogen
Q87: What is the [AsO<sub>4</sub><sup>3-</sup>] for a solution