Multiple Choice
What is the hydronium ion concentration of a 0.500 mol L-1 aqueous acetic acid solution with Ka = 1.8 × 10-5?
The equation for the dissociation of acetic acid is below: 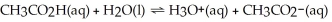
A) 3.0 × 10-2 mol L-1
B) 4.2 × 10-2 mol L-1
C) 3.0 × 10-3 mol L-1
D) 4.2 × 10-3 mol L-1
Correct Answer:

Verified
Correct Answer:
Verified
Q1: What is the pH of a 0.250
Q2: HNO<sub>3</sub> is a strong acid.
Q4: For Na<sub>2</sub>CO<sub>3</sub>,predict whether the aqueous solution is
Q5: A certain acid,HA,has a K<sub>a</sub> given by:<br>HA
Q6: Complete the following equation.List the conjugate acid
Q7: Which one of the following salts,when dissolved
Q8: What is the pH of a 0.563
Q9: Hypochlorous acid (HOCl)has an ionization constant of
Q10: A solution with a hydroxide ion concentration
Q11: Calculate the pH of a 0.080 mol