Multiple Choice
A 7.0 × 10-3 mol L-1 aqueous solution of 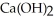 At 25.0 °C has a pH of:
At 25.0 °C has a pH of:
A) 12.15
B) 1.85
C) 1.4 × 10-2
D) 7.1 × 10-13
E) 11.85
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q90: For NH<sub>3</sub>,predict whether a 1 M aqueous
Q91: In which of the following cases is
Q92: The definition of a neutralization reaction as
Q93: A saturated aqueous solution of calcium hydroxide
Q94: What is the pH of a 0.470
Q96: In the following reversible reaction the Br∅nsted
Q97: For which of the following polyprotic acids
Q98: Which one of the following salts,when dissolved
Q99: Which of the following are Lewis bases?<br>I.BCl<sub>3
Q100: For NaCl,predict whether the aqueous solution is