Multiple Choice
What is the pH of a 0.30 mol L-1 pyridine solution that has Kb = 1.9 × 10-9? The equation for the dissociation of pyridine is below:
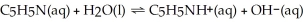
A) 4.62
B) 8.72
C) 9.38
D) 10.38
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q63: Amine bases are known as strong bases.
Q64: Calculate the pH for an aqueous solution
Q65: Choose the Br∅nsted-Lowry acids and bases in
Q66: The acid-dissociation constant at 25.0 °C for
Q67: What is the indication of the relative
Q69: Which of the following statements concerning aqueous
Q70: List the following acids in order of
Q71: What would be the pH of a
Q72: What is the pH of a 0.240
Q73: The first ionization step is approximately 100%