Multiple Choice
The acid-dissociation constant of hydrocyanic acid (HCN) at 25.0 °C is 4.9 × 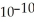
.What is the pH of an aqueous solution of 0.080 mol L-1 sodium cyanide (NaCN) ?
A) 11.11
B) 2.89
C) 1.3 × 10-3
D) 7.8 × 10-12
E) 3.9 × 10-11
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q110: What is the [Cl<sup>-</sup>] of a solution
Q111: pOH = 3.14 is equivalent to:<br>A)pH =
Q112: The term pH = -ln [H<sup>+</sup>].
Q113: For HI,predict whether the solution is acidic,basic
Q114: Choose the Br∅nsted-Lowry acids and bases in
Q116: Which indication of relative acid strengths is
Q117: Calculate the pH of a 1.60 mol
Q118: Calculate the pH of a 0.800 mol
Q119: A solution has pOH of -0.47.This means
Q120: According to the Arrhenius theory,a neutralization reaction