Multiple Choice
Calculate the pH of a 0.60 mol L-1 aqueous H2SO3 solution that has the stepwise dissociation constants Ka1 = 1.5 × 10-2 and 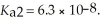
A) 1.02
B) 1.06
C) 1.82
D) 2.04
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q70: List the following acids in order of
Q71: What would be the pH of a
Q72: What is the pH of a 0.240
Q73: The first ionization step is approximately 100%
Q74: What is the [HPO<sub>4</sub><sup>-2</sup>] of a solution
Q76: 0.653 g of a monoprotic acid (MW=
Q77: Which Bronsted-Lowry acid is not considered to
Q78: The base-dissociation constant of ethylamine ( <img
Q79: What is the pH of an aqueous
Q80: Choose the Br∅nsted-Lowry acids and bases in