Multiple Choice
What is the pH of a 0.40 mol L-1 aqueous H2Se solution that has the stepwise dissociation constants Ka1 = 1.3 × 10-4 and 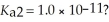
A) 2.14
B) 3.89
C) 4.28
D) 5.57
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q54: What is the pH of a 0.253
Q55: Which of the following are Br∅nsted-Lowry acids?<br>I.CH<sub>3</sub>COOH<br>II.[Cu(H<sub>2</sub>O)<sub>4</sub>]<sup>2+
Q56: If an equal number of moles of
Q57: What is the [K<sup>+</sup>] of a solution
Q58: 0.272 g of a monoprotic acid (MW
Q60: What is the pH of a 0.020
Q61: A 0.0925 g sample of a monoprotic
Q62: If one mole of Ba(OH)<sub>2</sub> is added
Q63: Amine bases are known as strong bases.
Q64: Calculate the pH for an aqueous solution