Multiple Choice
Calculate the pH of an aqueous solution that is 0.210 mol L-1 in nitrous acid ( 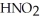 ) and 0.290 mol L-1 in potassium nitrite (
) and 0.290 mol L-1 in potassium nitrite ( 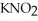 ) .The acid dissociation constant of nitrous acid is 4.50 ×
) .The acid dissociation constant of nitrous acid is 4.50 × 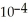 .
.
A) 3.487
B) 3.210
C) 13.86
D) 10.51
E) 4.562
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q9: An acid has a K<sub>a</sub> = 1
Q10: What is the pH of an aqueous
Q11: What is the [CH<sub>3</sub>COO<sup>-</sup>]/[CH<sub>3</sub>COOH] ratio necessary to
Q12: Which among the following pairs is inefficient
Q13: Twenty-five milliliters of 0.10 M HCl(aq)is titrated
Q15: Determine the [F<sup>-</sup>] of the following aqueous
Q16: The pH of a buffer solution changes
Q17: How will addition of sodium chloride affect
Q18: What is the pH of a 0.30
Q19: Which of the following statements correctly describe