Multiple Choice
Calculate the percent ionization of nitrous acid in an aqueous solution that is 0.249 mol L-1 in nitrous acid.The acid dissociation constant of nitrous acid is 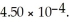
A) 1.12 × 10-4
B) 0.0450
C) 4.25
D) 0.342
E) 5.53
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q16: The pH of a buffer solution changes
Q17: How will addition of sodium chloride affect
Q18: What is the pH of a 0.30
Q19: Which of the following statements correctly describe
Q20: Calculate the pH of an aqueous solution
Q22: What is the pH of an aqueous
Q23: A buffer is 0.282 M C<sub>6</sub>H<sub>5</sub>COOH(aq)and 0.282
Q24: Ten milliliters of 0.10 M NH<sub>3</sub>(aq)(K<sub>b</sub> =
Q25: What is the pH at the equivalence
Q26: What is the pH of an aqueous