Multiple Choice
What is the pH of an aqueous buffer solution that is 0.211 mol L-1 in lactic acid and 0.111 mol L-1 in sodium lactate? The 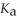 Of lactic acid is 1.4 ×
Of lactic acid is 1.4 × 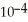 .
.
A) 14.28
B) 10.43
C) 5.48
D) 3.57
E) 4.13
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q106: What is the pH of a solution
Q107: 25 ml of 0.10 M aqueous acetic
Q108: The pH of a buffer depends mainly
Q109: Determine the pH of the following aqueous
Q110: In 0.100 M HC<sub>2</sub>H<sub>3</sub>O<sub>2</sub>(aq),[H<sub>3</sub>O<sup>+</sup>(aq)] = [C<sub>2</sub>H<sub>3</sub>O<sub>2</sub><sup>-</sup>(aq)] =
Q112: Twenty-five milliliters of 0.10 M HCl(aq)is titrated
Q113: Phenol red indicator changes from yellow to
Q114: In a solution prepared by mixing equal
Q115: It is easier to calculate the pH
Q116: In the titration of a solution of